





INDICATIONS AND USAGE
ABSORICA and ABSORICA LD (isotretinoin) capsules are indicated for the treatment of severe recalcitrant nodular acne in non-pregnant patients 12 years of age and older with multiple inflammatory nodules with a diameter of 5 mm or greater. Because of significant adverse reactions associated with their use, ABSORICA and ABSORICA LD are reserved for patients with severe nodular acne who are unresponsive to conventional therapy, including systemic antibiotics.
Limitations of Use:
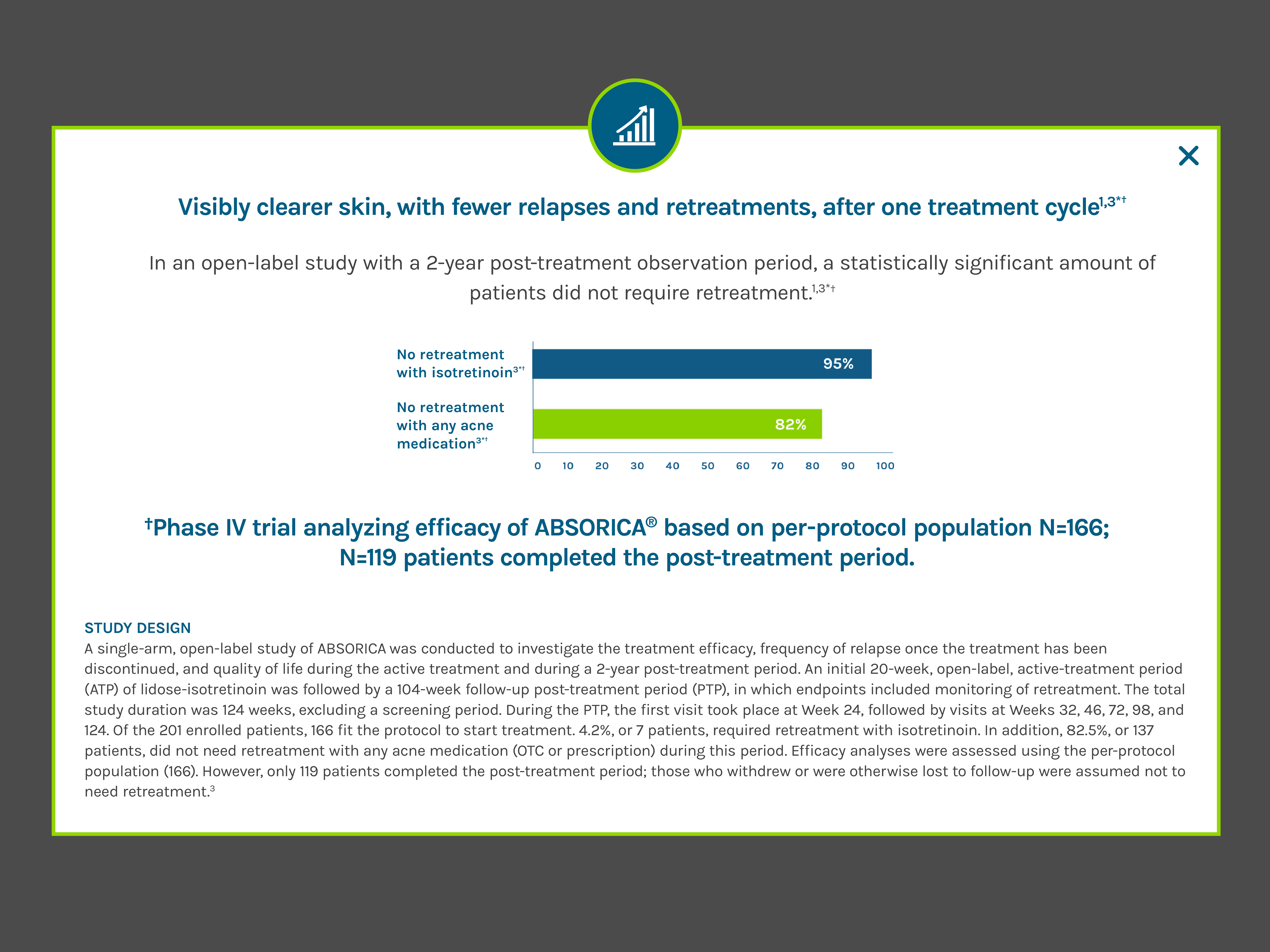
If a second course of ABSORICA or ABSORICA LD therapy is needed, it is not recommended before a two-month waiting period because the patient’s acne may continue to improve following a 15 to 20-week course of therapy
IMPORTANT SAFETY INFORMATION
WARNING: EMBRYO-FETAL TOXICITY – CONTRAINDICATED IN PREGNANCY
ABSORICA and ABSORICA LD can cause severe life-threatening birth defects and is contraindicated in pregnancy. There is an extremely high risk that severe birth defects will result if pregnancy occurs while taking any amount of ABSORICA or ABSORICA LD even for short periods of time. Potentially any fetus exposed during pregnancy can be affected. There are no accurate means of determining prenatally whether an exposed fetus has been affected. If pregnancy occurs, discontinue ABSORICA and ABSORICA LD immediately and refer the patient to an Obstetrician-Gynecologist experienced in reproductive toxicity for further evaluation and counseling.
Because of the risk of embryo-fetal toxicity, ABSORICA and ABSORICA LD are available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the iPLEDGE® REMS.
CONTRAINDICATIONS
Pregnancy: ABSORICA and ABSORICA LD are contraindicated in pregnancy
Hypersensitivity: ABSORICA and ABSORICA LD are contraindicated in patients with hypersensitivity to isotretinoin (or Vitamin A, given the chemical similarity to isotretinoin) or to any of its components (anaphylaxis and other allergic reactions have occurred)
WARNINGS AND PRECAUTIONS
ABSORICA and ABSORICA LD are Not Substitutable: The bioavailability and the recommended dosage of ABSORICA and ABSORICA LD are different. For example, while ABSORICA and ABSORICA LD both have a 20 mg strength, these strengths have different bioavailability and are not substitutable.
Psychiatric Disorders: ABSORICA and ABSORICA LD may cause depression, psychosis and, rarely, suicidal ideation, suicide attempts, suicide, and aggressive and/or violent behaviors. Prior to and during therapy, assess for these conditions.
Patients should immediately stop ABSORICA and ABSORICA LD and promptly contact their prescriber if they develop depression, mood disturbance, psychosis, or aggression. Discontinuation of ABSORICA and ABSORICA LD may be insufficient; further evaluation may be necessary such as a referral to a mental healthcare professional.
Intracranial Hypertension (Pseudotumor Cerebri): Isotretinoin use has been associated with cases of intracranial hypertension (pseudotumor cerebri), some of which involved concomitant use of tetracyclines. Concomitant treatment with tetracyclines should therefore be avoided with ABSORICA and ABSORICA LD use.
Serious Skin Reactions: There have been postmarketing reports of erythema multiforme and severe skin reactions [e.g., Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN)] associated with isotretinoin use. These reactions may be serious and result in death, life-threatening events, hospitalization, or disability. Patients should be monitored closely for severe skin reactions, and ABSORICA and ABSORICA LD should be discontinued if they occur.
Acute Pancreatitis: Acute pancreatitis has been reported with isotretinoin use in patients with either elevated or normal serum triglyceride levels. In rare instances, fatal hemorrhagic pancreatitis has been reported. If symptoms of pancreatitis occur, the patient should discontinue ABSORICA and ABSORICA LD and seek medical attention.
Lipid Abnormalities: Elevations of serum triglycerides above 800 mg/dL have been reported with isotretinoin use. These lipid changes were reversible upon isotretinoin capsule cessation. Some patients have been able to reverse triglyceride elevation by reduction in weight and restriction of dietary fat and alcohol while continuing isotretinoin or through dosage reduction. The cardiovascular consequences of hypertriglyceridemia associated with isotretinoin are unknown.
Hearing Impairment: Impaired hearing has been reported in patients taking isotretinoin; in some cases, the impairment has been reported to persist after therapy has been discontinued. Mechanism(s) and causality for this reaction have not been established. Patients who experience tinnitus or hearing impairment should discontinue ABSORICA or ABSORICA LD treatment and be referred for specialized care for further evaluation.
Hepatotoxicity: Clinical hepatitis has been reported with isotretinoin use. Additionally, mild to moderate elevations of liver enzymes have been observed in approximately 15% of individuals treated during clinical trials with isotretinoin capsules, some of which normalized with dosage reduction or continued administration of the drug. If normalization does not readily occur or if hepatitis is suspected during treatment, ABSORICA and ABSORICA LD should be discontinued.
Inflammatory Bowel Disease: Isotretinoin has been associated with inflammatory bowel disease (including regional ileitis) in patients without a prior history of intestinal disorders. In some instances, symptoms have been reported to persist after isotretinoin treatment has been stopped. Patients experiencing abdominal pain, rectal bleeding or severe diarrhea should discontinue ABSORICA or ABSORICA LD immediately.
Musculoskeletal Abnormalities: Effects of multiple courses of isotretinoin on the developing musculoskeletal system are unknown. There is some evidence that long-term, high-dose, or multiple courses of therapy with isotretinoin have more of an effect than a single course of therapy on the musculoskeletal system. It is important that ABSORICA and ABSORICA LD be given at the recommended dose for no longer than the recommended duration.
Ocular Abnormalities: Visual problems should be carefully monitored. If visual difficulties occur, the patient should discontinue ABSORICA and ABSORICA LD treatment and obtain an ophthalmological examination.
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5%) are: dry lips, dry skin, back pain, dry eye, arthralgia, epistaxis, headache, nasopharyngitis, chapped lips, dermatitis, increased creatine kinase, cheilitis, musculoskeletal discomfort, upper respiratory tract infection, reduced visual acuity.
DRUG INTERACTIONS
Vitamin A: ABSORICA and ABSORICA LD are closely related to vitamin A. Therefore, the use of both vitamin A and ABSORICA or ABSORICA LD at the same time may lead to vitamin A related adverse reactions. Patients treated with ABSORICA and ABSORICA LD should be advised against taking supplements containing Vitamin A to avoid additive toxic effects.
Tetracyclines: Concomitant treatment with ABSORICA and ABSORICA LD and tetracyclines should be avoided because isotretinoin use has been associated with a number of cases of intracranial hypertension (pseudotumor cerebri), some of which involved concomitant use of tetracyclines
USE IN SPECIFIC POPULATIONS
There are no data on the presence of isotretinoin in either animal or human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for serious adverse reactions in nursing infants from isotretinoin, advise patients that breastfeeding is not recommended during treatment with ABSORICA and ABSORICA LD, and for at least 8 days after the last dose of ABSORICA or ABSORICA LD.
These are not all of the possible side effects of ABSORICA and ABSORICA LD. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or Sun Pharmaceutical Industries, Inc. at 1-800-818-4555.